CDC approves Pfizer-BioNTech booster shot
Pfizer to be first to rollout vaccine booster
September 28, 2021
WEB EXCLUSIVE The Center For Disease Control and Prevention approved the Pfizer-BioNTech booster shot on Sept. 22 for individuals 65 years or older, people over the age of 18 with underlying medical conditions and for those who work in a high-risk setting.
“Obviously none of us can get it because we are too young, but I think having our grandparents and family members have access to this is important in so many ways,” sophomore Brennan Damond said. “It benefits them because they are exposed to so many new people every day, but within our direct school community I do not think anything will change very much.”
Those eligible will need to have received their vaccination at least six months before they receive their booster, according to the CDC website.
“Our school did a really wonderful job staying in close contact with medical professionals, researchers and CDC recommendations for schools,” LIFE Coordinator Bryan Lorentz said. “We are constantly looking for guidance and thoroughly looking through any new information that is coming across our desks and then consulting the professionals.”
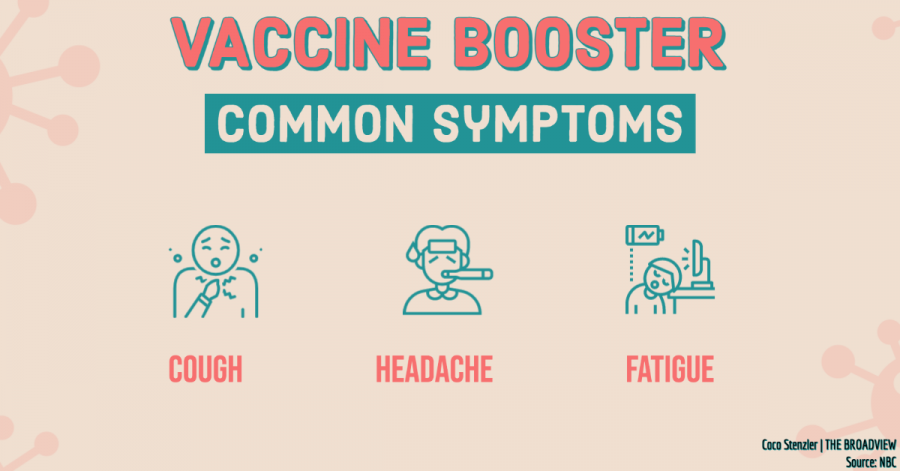
Symptoms of the Pfizer-BioNTech booster shot are similar to the symptoms of the second vaccine dose such as arm pain, fatigue, and headaches, according to NBC.
“I think this third dosage will be great for our community,” senior Bridget Mills said. “I hope that with this booster shot we will be able to participate in more all-school gatherings and events.”
At this point in time, the only vaccine booster that has been approved is produced by Pfizer-BioNTech. Individuals who received the Moderna or Johnson & Johnson vaccines will most likely need a booster shot as well, according to the CDC website.
“The school has been doing a great job monitoring everyone through the pandemic,” Damond said. “The booster shot will help us and keep the whole community safe.”